Titrating with bottle-top burettes
Titration is a volumetric method used for the quantitative analysis of a dissolved substance.
How to titrate?
Using a bulb pipette, a defined portion of a sample (liquid with an unknown fraction of dissolved material, e.g., acetic acid) is placed in an Erlenmeyer flask.
After dilution with water, 3 drops of an indicator solution are added. Then, with continuous swirling, a suitable titrant of known concentration (e.g., 0.1 M NaOH) is added from a burette until a color change in the indicator signals the endpoint of the titration.
Using the chemical equation and the volume of titrants used, the amount of substance dissolved in the sample can be calculated.


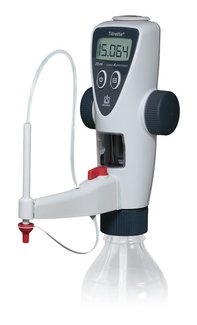
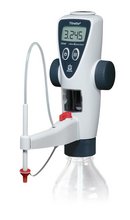
Functional principle of bottle-top burettes
Bottle-top burettes are mounted directly upon the reservoir bottle. By the upward movement of the piston, liquid is aspirated from the reagent bottle into the burette cylinder. By the subsequent downward movement of the piston, the liquid is released slowly, and added to the sample through the discharge tube until the titration is finished, e.g., by change of color.
Titration with the Titrette® from BRAND
The piston moves when the hand wheels are turned, and this takes up or discharges the liquid. The electronics of the instrument automatically recognize the direction of rotation, whether filling or titration is taking place. The liquid can be taken in quickly, and can then be delivered exactly, very slowly, drop by drop. A recirculation valve makes it possible to run the liquid back into the bottle during priming. Thus, air bubbles can be removed without loss of medium. The instrument can readily be disassembled in the laboratory for cleaning and maintenance.

Curious?
Documents download
It can be used in many applications for aqueous and non-aqueous solutions (e.g., alcoholic KOH) up to 1 M.
Parts that come into contact with liquid are made from various specially resistant materials, e.g., borosilicate glass, PTFE, platinum-iridium, Al2O3 ceramic.